NDTV Media, India's best-known and most respected Media Marketing & Consulting Company has been appointed by UFO Moviez India Ltd. as the sole & exclusive sales partner for their Digital Cinema Advertising Business in the Private Sector. UFO Moviez India Ltd. has the largest digital cinema network in the world with more than 1200 installations screens across India.
As a part of this arrangement, NDTV Media will be responsible for selling the advertising at the cinema theatres for which UFO Moviez India Ltd. has the advertising rights. This would include the selling of airtime on the cinema screens as well as on ground activation at these cinema halls, which will include seat branding, ticket branding, putting up standees & stalls and other ground activation for advertisers.
Speaking on this appointment, Niraj Dutt, Chief Operating Officer, NDTV Media Ltd. said, "We are extremely pleased to partner with UFO Moviez India Ltd. on this pioneering initiative. Digital Cinema is a powerful innovation in the space of cinema advertising and brings with it a host of benefits including flexibility, reach and transparency. With a satellite based delivery mechanism, Digital Cinema offers the opportunity to advertisers to reach cinema halls across the country in multiple languages with just one Digi-Beta. For the first time, this format allows for theatre-wise and spot-wise monitoring of the client's activity".
He further added, "After having a stronghold in the Television and Internet space, Digital Cinema is another medium that NDTV Media is venturing into thereby being able to offer its clients multiple touch points to reach its consumers. In keeping with the expansion plan that we have at NDTV Media, Digital Cinema Advertising provides a new platform which we can offer our clients. We are confident that this relationship will be mutually beneficial."
Rajesh Mishra, CEO - India Operations, UFO Moviez India Ltd. said, "Earlier the single screens were largely unable to monetize the advertising opportunities in their cinemas owing to the fragmented nature of the market. Now that we have reached 1200 screens across India, we can begin to tap the advertising opportunities that are possible with our network of cinemas. Their handling of the ad sales activity will allow us to focus on our core business of Digital Cinema Operations. NDTV Media will bring to the table their extensive and specialized expertise and contacts in the field of advertising. We look forward to a long association with NDTV Media."
About NDTV Media
NDTV Media is India's best-known and most respected Media Marketing and Consulting Company. NDTV Media started operations in the year 2002 and is now among the leading revenue generators in the Indian advertising industry in the television and internet sector. The company is led by Raj Nayak, a media professional, known in the Indian media circles for his vision and foresight.
With a 1500+ client list across all brands, the largest client count for any independent media marketing company in India, NDTV Media's current bouquet of offerings in the media space include NDTV 24X7, NDTV India, NDTV Profit, NDTV Good Times, NDTV MetroNation, Mi Marathi, Just TV Punjabi and Sahara's Entertainment channels viz., Sahara One, Filmy, Firangi. In addition to this NDTV Media is also the exclusive sales representative for MSN in India and for Microsoft Advertising platform which includes all the popular sites like Facebook, Bollywood Hungama, HDFC Securities, Equitymaster, etc. and is responsible for all revenue generating efforts for the MSN offerings and for the online and mobile verticals under Microsoft Advertising.
Monday, June 30, 2008
Castrol to Become an Official Sponsor of FIFA World Cup
 Castrol has strengthened its position in football by signing a prestigious six-year deal as a FIFA World Cup Sponsor until 2014.
Castrol has strengthened its position in football by signing a prestigious six-year deal as a FIFA World Cup Sponsor until 2014.The agreement awards the global lubricants company worldwide rights for the 2010 FIFA World CupT in South Africa, the 2014 FIFA World CupT in Brazil and the two FIFA Confederations Cups which fall within the 2007-2014 period.
The news comes following the company's successful sponsorship of the recent European championships - the UEFA EURO2008 - which saw Castrol develop the Castrol Performance Index, a new tool which helps football fans to objectively analyse individual player and team performances.
"Our investment in football has proved a tremendous success and allowed Castrol to develop new opportunities in a way that has added value to our business partners; excited and rewarded our fans; and motivated our staff," said Senior Vice President for Lubricants, Mike Johnson.
Commenting on the announcement, FIFA President Joseph S. Blatter, said "With Castrol, we are delighted to welcome a committed football supporter into our global sponsorship family. The FIFA World Cup, with more than 26 billion TV viewers, attracts massive involvement from fans and non-fans alike, who are drawn to the drama and excitement that comes with top national-team football. With its new Performance Index, Castrol has enhanced the fans' experience with exclusive data on the players' speed, efficiency and performance in matches. The index was developed with renowned football experts such as Arsène Wenger, Ottmar Hitzfeld and Emilio Butragueño and it underlines Castrol's passion for the game,"
"This partnership not only highlights FIFA's confidence in South Africa and Brazil as the host nations, but also shows that the global business community believes in the incredibly positive impact the events will have," Blatter added.
Through the creation of the Castrol Performance Index, Castrol was able to showcase the importance of the brand's core values of passion, technical progress and performance in a way that was both innovative and engaging.
Also commenting on the deal, Castrol ambassador Arsene Wenger said "I chose to work with Castrol because its football sponsorship went beyond simply ticket promotions and branding. The development and launch of a new performance index was something which really interested me, and I was impressed by the way they used their experience in improving vehicle performance to champion the objective measurement of players and teams."
The FIFA World CupT sponsorship is the biggest in the company's 100-year history and will help Castrol continue to modernise its brand to deliver even more powerful and relevant offers for its consumers, which include a simplified product range and new technology benefits.
The connection with events such as the 2010 FIFA World CupT in South Africa and the 2014 FIFA World CupT in Brazil provide a route to a mass football audience that overlaps strongly with the brand's 45 million consumer base. Football fans can look forward to Castrol and FIFA making further announcements about concepts related to their new partnership later this year.
Frost & Sullivan Expects the Engineering Services Outsourcing Market (ESO) to Touch US $40 Billion by 2015
India holds a relatively strong position in the global Engineering Services Outsourcing (ESO) market, with a potential of US $40 billion by 2015. India's strengths lie in the automotive, aerospace and hi-tech telecom sectors accompanied by a large talent pool and experience in this field. The growth in engineering services signifies the need for global companies to expand their R&D centers beyond their home countries.
Another attractive factor for these global manufacturers is low labor cost along with quality, one of the main reasons that makes India a major outsourcing destination.
Indian engineering service providers are technically proficient and have a fervent eye for detail ensuring that service levels remain better than competing countries. Most of the Indian engineering services providers are at par with competition and offer an array of services, which include computer aided design (CAD), computer aided manufacturing (CAM), computer aided engineering (CAE) while vertical specific engineering services companies provide product life cycle management solutions, reengineering and design change management, too.
To target the maximum potential from engineering service providers and automotive manufacturers, there is a need to address key issues like quality manpower, low cost services and put India on the global market as an engineering service provider. Frost & Sullivan is organizing a two-day high-powered MindXchange to address these issues through its summit "Opportunities in the Automotive Engineering Services Outsourcing Market" to be held on the 11th and 12th August, 2008 at the Leela, Goa.
The summit seeks to understand and discuss the growth needs of companies in the engineering services outsourcing as well as the automotive industry by fostering common understanding of business needs and opportunities that could lead to business partnerships. This forum endeavors to nurture strategic business decisions by bringing together key decision makers and thought leaders from the domestic and international automotive industry and engineering services companies.
Eminent speakers such as, Dr. Arun Jaura, Mahindra & Mahindra; Tarak Balaji, Delphi; Vivek Narayanan, Pierburg; S. D. Pradhan, Argentum Motors; and senior representatives from Satyam Ventures, BMW and many more are expected to be present at the summit.
Satyam Ventures is the event partner and Auto Monitor, Autocar Professional, Dataquest and The Machinist are the media partners for the event.
About Frost & Sullivan:
"We Accelerate Growth" - Frost & Sullivan
Frost & Sullivan, the Growth Partnership Company, partners with clients to accelerate their growth. The company's TEAM Research, Growth Consulting and Growth Team Membership empower clients to create a growth-focused culture that generates, evaluates and implements effective growth strategies. Frost & Sullivan employs over 45 years of experience in partnering with Global 1000 companies, emerging businesses and the investment community from more than 30 offices on six continents. For more information about Frost & Sullivan's Growth Partnerships, visit http://www.frost.com.
Another attractive factor for these global manufacturers is low labor cost along with quality, one of the main reasons that makes India a major outsourcing destination.
Indian engineering service providers are technically proficient and have a fervent eye for detail ensuring that service levels remain better than competing countries. Most of the Indian engineering services providers are at par with competition and offer an array of services, which include computer aided design (CAD), computer aided manufacturing (CAM), computer aided engineering (CAE) while vertical specific engineering services companies provide product life cycle management solutions, reengineering and design change management, too.
To target the maximum potential from engineering service providers and automotive manufacturers, there is a need to address key issues like quality manpower, low cost services and put India on the global market as an engineering service provider. Frost & Sullivan is organizing a two-day high-powered MindXchange to address these issues through its summit "Opportunities in the Automotive Engineering Services Outsourcing Market" to be held on the 11th and 12th August, 2008 at the Leela, Goa.
The summit seeks to understand and discuss the growth needs of companies in the engineering services outsourcing as well as the automotive industry by fostering common understanding of business needs and opportunities that could lead to business partnerships. This forum endeavors to nurture strategic business decisions by bringing together key decision makers and thought leaders from the domestic and international automotive industry and engineering services companies.
Eminent speakers such as, Dr. Arun Jaura, Mahindra & Mahindra; Tarak Balaji, Delphi; Vivek Narayanan, Pierburg; S. D. Pradhan, Argentum Motors; and senior representatives from Satyam Ventures, BMW and many more are expected to be present at the summit.
Satyam Ventures is the event partner and Auto Monitor, Autocar Professional, Dataquest and The Machinist are the media partners for the event.
About Frost & Sullivan:
"We Accelerate Growth" - Frost & Sullivan
Frost & Sullivan, the Growth Partnership Company, partners with clients to accelerate their growth. The company's TEAM Research, Growth Consulting and Growth Team Membership empower clients to create a growth-focused culture that generates, evaluates and implements effective growth strategies. Frost & Sullivan employs over 45 years of experience in partnering with Global 1000 companies, emerging businesses and the investment community from more than 30 offices on six continents. For more information about Frost & Sullivan's Growth Partnerships, visit http://www.frost.com.
Global Real Estate Transparency Increasing in Nearly Half of Countries Rated According to Jones Lang LaSalle Index
 Emerging markets have significantly improved their levels of real estate transparency, according to the latest Global Real Estate Transparency Index ('Index') from Jones Lang LaSalle, a professional services firm specialising in real estate, and LaSalle Investment Management, its global real estate investment management subsidiary.
Emerging markets have significantly improved their levels of real estate transparency, according to the latest Global Real Estate Transparency Index ('Index') from Jones Lang LaSalle, a professional services firm specialising in real estate, and LaSalle Investment Management, its global real estate investment management subsidiary.In some countries, particularly in emerging markets, notable differences can be observed in transparency not only between cities, but within cities as well.
Anuj Puri, Chairman and Country Head India, Jones Lang LaSalle Meghraj says,, "In India, the issue of real estate transparency at a sub-national city level is gaining importance as real estate investors and corporate occupiers extend into new regions in their search for higher returns or cost-effective locations. International investors and occupiers are moving into secondary and tertiary cities that have not been traditional targets of the real estate community."
The Index, which provides a rigorous framework for comparing the level of real estate transparency in 82 markets around the world, shows that nearly half of the countries surveyed in 2006 demonstrated a significant improvement in their transparency score two years later. Transparency levels globally are improving as governments seek to streamline regulatory and legal hurdles to aid cross-border movement of capital and corporate facilities. Only Venezuela posted a lower transparency score this year compared with 2006, principally due to changes in government regulations and new taxation policies targeting foreign investors.
In keeping with historical results, the Australian and US real estate markets remain among the most transparent in the world. With the addition of new variables relating to the quality and frequency of valuations, service charge transparency and financing transparency, Canada now ranks as the world's most transparent commercial real estate market (see Table 1).
Jacques Gordon, Global Strategist at LaSalle Investment Management says, "The steady improvement in transparency, particularly over the last four years, is closely linked to the forces of globalisation that drive investors to move across borders in search of higher risk-adjusted returns. This movement of both capital and corporations around the world has created an even greater need for information about markets. It has also created an incentive for governments to streamline bureaucratic practices which prevent the free flow of capital into and out of global markets."
"Many cross-border investors focus on more mature, open and transparent real estate markets such as the UK, Canada, Netherlands and Hong Kong. However, opportunistic investors will consider the emerging, less mature, less open and semi-transparent markets, but will require higher returns to compensate for the higher risks associated with lower transparency. Only the most opportunistic investors will consider semi-transparent markets found in Eastern Europe, Latin America and Southeast Asia. Opaque markets, such as Algeria, Belarus and Cambodia, are still very problematic, from the perspective of both investors and corporate occupiers."
"Overall, increases in the free flow of capital, tenants, management and market information are closely coupled with the rising productivity of real estate in countries with improving transparency. This productivity can be measured by investment returns and in terms of corporations' ability to provide efficient workspaces, logistics facilities, retail environments, and business and leisure centres for travellers and domestic markets alike. While many emerging markets have made improvements to their real estate transparency, our index shows that not all governments and market participants have embraced the necessary changes."
The Index shows that 28 countries posted transparency scores that were within 10 basis points of their scores in 2006. Jones Lang LaSalle researchers say that consistency in transparency scores over the years is expected in real estate markets such as Australia, the UK, the US, Singapore and Hong Kong where a high degree of transparency already exists.
However, countries such as Argentina, Greece, Indonesia, and Peru have consistently scored in the low transparency range over the last few years despite an increase in cross-border trade, finance and commerce over the same time period.
A number of countries in the 'frontier markets' have been included in the Index for the first time, with Belarus, Sudan, Algeria, Cambodia and Syria scored as 'opaque'. Other new entrants into the Index, Bahrain, Bulgaria, Estonia, Latvia, Croatia, Abu Dhabi, and Lithuania, scored in the 'semi-transparent' range, while Oman, Qatar, Morocco, Kuwait, Pakistan and Kazakhstan all scored in the 'low transparency' range.
Asia Pacific Transparency
In Asia Pacific, transparency has improved across most of the region since 2006; however, the gains are relatively modest for most countries, particularly when compared with the larger improvements between 2004 and 2006, and the more recent gains in Europe and Middle East and North Africa.
The biggest improvers in Asia Pacific are India, China and Vietnam, all of which have received considerably greater attention from investors and corporate occupiers in recent years. China exhibited the greatest improvement, moving up to the semi-transparent tier (Tier 3) and is now considered more transparent than India. Notwithstanding this, the two emerging giants of the region remain very close in terms of overall real estate transparency.
Dr Jane Murray, Head of Research, Asia Pacific at Jones Lang LaSalle says, "Where improvements are observed, a key reason has been the increased availability of data on major market fundamentals, particularly for the office sector. The growth of the REIT sector has also had a further positive impact on improving financial disclosures across the region, building on gains observed between 2004 and 2006. In relation to the impact of government, the processes surrounding compulsory acquisition of real estate by the public sector have also become more transparent over the two years."
"At the other end of the spectrum, there is room for improvement in the availability of indices relating to the investment performance of real estate assets, and those based on unlisted real estate vehicles. As Pan Asian real estate funds become more prominent within global investment portfolios and institutional ownership increases in developing markets, more indices should become available.
Some regulatory factors, such as the consistent application of taxes, building and planning codes, and enforceability of contracts, remain areas of concern in a number of markets. Aspects of the real estate transaction process also remain wanting in some countries," adds Dr Murray.
"It is important to note that transparency enhancements should not be viewed as the sole determinant of regional capital flows, instead market fundamentals appear to be the main driving force behind transaction volumes, as we can see from the solid increases in cross-border transaction volumes in Japan and Korea over the past two years, though the two countries recorded minimal improvements in transparency. Nevertheless, higher transparency is resulting in a more dispersed flow of capital between countries across the region. It is therefore noteworthy that most of the countries surveyed are continuing to improve on the transparency measures, and as a result, enhancing the attractiveness of these markets for real estate investors and occupiers," concludes Dr Murray.
The impact of the additional criteria included in the 2008 Transparency Index is typically a minor fall in the score for the more transparent markets and a minor improvement in the score for the less transparent markets. For the composite score, which includes the additional criteria, Hong Kong and Singapore moved slightly down to the top of the transparent tier. However, the overall ranking for countries is not impacted when the additional measures are included in the Index.
Jones Lang LaSalle warns that a high level of transparency does not eliminate risk or guarantee a strong investment return or efficient corporate transaction. High transparency does not prevent market swings as the most transparent commercial real estate investment vehicles - listed real estate securities - can also exhibit volatility.
The 2008 Real Estate Transparency Index added several new dimensions and the addition of 26 new countries. New transaction process measurements were included for debt financing commitment terms and fees, occupier service charges, and facilities and project management services and fees. The outcome of the transaction process measures may be helpful for corporations who need to understand total occupancy and transactions costs or institutional investors seeking to analyse broad market statistics and historical performance benchmarks.
HP ProCurve Launches Gigabit Solution for SMBs
 HP ProCurve Networking today launched two affordable Gigabit Ethernet switches for small and medium businesses (SMBs) that need increased network performance for large file transfers and high bandwidth applications such as graphical data, video streams, large databases, network backup and data storage. The standards-based ProCurve Switch 2510G Series comprises two fully-managed Layer 2 switches that offer Gigabit Ethernet connectivity.
HP ProCurve Networking today launched two affordable Gigabit Ethernet switches for small and medium businesses (SMBs) that need increased network performance for large file transfers and high bandwidth applications such as graphical data, video streams, large databases, network backup and data storage. The standards-based ProCurve Switch 2510G Series comprises two fully-managed Layer 2 switches that offer Gigabit Ethernet connectivity.The new plug-and-play switches are easy to deploy, manage and maintain for SMBs which typically do not have a large or specialized IT staff. Backed by ProCurve Lifetime Warranty, the 2510G Series is a cost-effective solution for SMBs whose existing networks are pushing the performance limits and which are seeking an upgrade to Gigabit connectivity to improve efficiency.
"ProCurve offers a choice of secure and affordable plug-and-play enterprise-grade networking solutions for SMBs that will enable them to expand their networks as their needs grow, said Louis Au, HP ProCurve Vice President and General Manager for Asia Pacific and Japan. "Our products are standards-based and engineered to the legendary HP quality."
ProCurve Switch 2510G Series Features & Benefits:
-- 24- and 48-port 10/100/1000 switches, each with SFP (small form-factor pluggable transceiver designed for Gigabit and Fast Ethernet) slots for optional fiber uplinks
-- Protected ports allow secure traffic segregation and control of network traffic
-- Secure network access to protect business assets by utilizing 802.1X, Web Authentication and MAC Authentication methods
-- Choice of ProCurve management interface includes an intuitive Web user interface, full-featured console access with Command line interface, and a centralized management option using ProCurve Manager
Pricing and Availability
The ProCurve Switch 2510G Series will be available from 1 July 2008 with the following pricing.
-- ProCurve Switch 2510G-24: INR 51,100
-- ProCurve Switch 2510G-48: INR 92,980
About HP ProCurve
HP ProCurve is the Network of Choice for best-in-class solutions, products and services for wired and wireless networks. ProCurve's Adaptive Networks vision enables customers to implement an open, standards-based network infrastructure that adapts to the changing needs of users, applications and organizations.
ProCurve was positioned in the Leaders quadrant in research and advisory firm Gartner, Inc.'s 2008 Magic Quadrant Report for Global Campus LANs and is No. 2 worldwide in Ethernet switch market revenue and ports according to Dell'Oro Group.
Fidelity Announces New Business Initiative in India
 Fidelity International today announced that it would launch its world-renowned, award winning, online fund platform, FundsNetworkT in India. FundsNetworkT will be an open architecture fund platform that will offer online a range of funds from a number of fund houses. It will help intermediaries to grow their business by allowing them to focus on customer acquisition, advice and relationship management without being concerned about back office and administration issues. India will be the 5th country in the Fidelity Group to launch FundsNetworkT, after the U.S., U.K., Germany and Taiwan. In Phase 1, Fidelity today launched the Fidelity Advisers Institute, Fidelity's centre for excellence, for advisers.
Fidelity International today announced that it would launch its world-renowned, award winning, online fund platform, FundsNetworkT in India. FundsNetworkT will be an open architecture fund platform that will offer online a range of funds from a number of fund houses. It will help intermediaries to grow their business by allowing them to focus on customer acquisition, advice and relationship management without being concerned about back office and administration issues. India will be the 5th country in the Fidelity Group to launch FundsNetworkT, after the U.S., U.K., Germany and Taiwan. In Phase 1, Fidelity today launched the Fidelity Advisers Institute, Fidelity's centre for excellence, for advisers.Robert Higginbotham, President, Fidelity International, said, "I am delighted that we are bringing our best-in-class online fund platform to India. Backed by our experience in international markets, FundsNetworkT will be a business partner for mutual fund agents (MFAs) by providing them Business Coaching to help them grow their business aided by state-of-the-art Practice Management tools. India is a strategic market for us and the FundsNetworkT initiative demonstrates our commitment to expanding the mutual fund industry here."
FundsNetworkT is a technology powered solution that allows MFAs to devote their time entirely to their professional role of managing and advising their clients. Advisers will benefit from business tools that will support transactional and reporting requirements as well as planning and guidance needs.
Ashu Suyash, Managing Director and Country Head - India, Fidelity International, added, "Market development and penetration of mutual funds has been severely limited by the smaller number of agents selling funds, especially when compared to the insurance industry's strength in number of agents. The challenge has been further aggravated by poor acceptance of mutual funds among insurance agents.
"In the first phase of FundsNetworkT, we are launching the Fidelity Advisers Institute, Fidelity's centre for excellence, for advisers, which will focus on Business Coaching and Practice Management with programmes tailored to help MFAs grow their business. The Fidelity Advisers Institute will also seek to bring in new advisers by helping them learn about mutual funds and becoming registered agents with AMFI. I believe that our AMFI Certification Preparatory Module can help improve the percentage of candidates passing the AMFI exam. At the same time, MFAs who are currently selling mutual funds can also avail of other training modules that will help them upskill and upsell."
The Fidelity Advisers Institute will offer comprehensive training and development programmes for advisers free of charge. This will include both e-learning and face-to-face training from a panel of trainers on a range of subjects, including marketing, holistic financial skills and soft skills, while also helping them learn good business practices. Advisers can visit www.fundsnetwork.co.in to see the programme schedule and to register themselves for training courses which begin in July.
One of the highlights of the online training is the AMFI Certification Preparatory Module which can be used by anyone who wants to become an adviser and is required to take the AMFI exam. This Module includes all 15 chapters in comprehensive detail and in an easy-to-understand format. It also provides mock exams that evaluate the candidates' preparedness and identify the topics that need more study.
About Fidelity
The word "fidelity" stands for commitment and integrity. It was for these values that it was chosen for the firm's name 60 years ago - we're committed to each and every customer and invest with integrity.
The recognition of this approach has enabled us to become one of the world's oldest, largest and leading fund managers¹ with almost US$1.8 trillion in global assets under management. FIL Limited manages more than US$260 billion worldwide and our US affiliate, Fidelity Management & Research LLC (FMR LLC) manages over US$1.5 trillion as at March 31, 2008.
The Fidelity story started in 1930 when the Fidelity Fund was founded in the United States (the 10th mutual fund founded in the US), and was followed by the formation of FMR LLC in 1946 to serve as investment adviser to this fund.
The focus on servicing each customer with individual commitment and integrity saw the firm grow and expand overseas. Today, Fidelity has offices in 25 countries around the globe and throughout Asia². FIL Limited was established in 1969 to serve the investment needs of individuals, institutions and advisers in markets outside the Americas. FIL Limited and its subsidiaries now manage assets of more than US$260 billion for investors worldwide and provide mutual funds, defined contribution pensions, segregated portfolios and multi-manager products.
Fidelity's widely acclaimed platform, FundsNetwork, has a presence in the U.S., U.K., Germany and Taiwan. Now India has become the latest country to get the FundsNetwork platform. In the U.K. FundsNetwork has $25 billion of third party administered assets and in the U.S., FundsNetwork has $766 billion of third party assets under management. (Both figures as at 31.12.07)
FIL Fund Management Private Limited (FFMPL) is the Indian arm of Fidelity International. It started its operations in the country in 2004 and currently offers Indian investors investment options through its five equity funds and four fixed income funds.
Central to Fidelity's success is a pioneering spirit, a commitment to innovation that sets new industry standards and an unmatched investment in research, talent, technology and investor education. And at the heart of our business philosophy is our belief in putting investors' interest above all else.
Thursday, June 19, 2008
123Greetings application now on Orkut
In a bid to add expression to emotions and make Social Networking Site 'Orkut' more expressive 123Greetings.com will now provide access to 20,000 free e-cards that are available for over 3,000 special occasions and events. 123greetings.com today announced the launch of a new application for Orkut.com where users will be able to see what cards other people in their network have sent, understand what cards are most popular and review the highest ranked cards by other Orkut users, in addition to sending as many ecards as they like.
Speaking on this development Mr. Arvind Kajaria, Founder of 123Greetings.com said "Orkut is catching up fast with Bebo, Facebook and MySpace. Users are looking for better ways to reach out to friends and make new friends. The '123Greetings Cards' application provides a simple-to-use interface that leverages the Web 2.0 features of Orkut.com with the depth and breadth of our massive collection of ecards. Most other applications feature a few hundred or a few thousand cards; we offer more than 20,000."
The "123Greetings Cards" application is also live on Bebo, Facebook, MySpace and Friendster. Mr. Kajaria went on to say, "The whole idea behind launching this ecard application was to provide more options for the users. Dedicated Orkut users can use Orkut's platform and 123Greeting's resources to send out their ecards. It makes card sending more enjoyable."
Speaking on this development Mr. Arvind Kajaria, Founder of 123Greetings.com said "Orkut is catching up fast with Bebo, Facebook and MySpace. Users are looking for better ways to reach out to friends and make new friends. The '123Greetings Cards' application provides a simple-to-use interface that leverages the Web 2.0 features of Orkut.com with the depth and breadth of our massive collection of ecards. Most other applications feature a few hundred or a few thousand cards; we offer more than 20,000."
The "123Greetings Cards" application is also live on Bebo, Facebook, MySpace and Friendster. Mr. Kajaria went on to say, "The whole idea behind launching this ecard application was to provide more options for the users. Dedicated Orkut users can use Orkut's platform and 123Greeting's resources to send out their ecards. It makes card sending more enjoyable."
Warwick and CII forge cutting edge research link
Warwick Manufacturing Group (WMGG), an international research organisation, is to boost its links with the Confederation of Indian Industry (CII) in order to boost cutting edge research in climate change, high-tech manufacturing and global healthcare, WMG announced Wednesday.
The two organisations will explore areas of cooperation and research collaboration in automotives, experiential engineering, robotics, healthcare, e-security, sustainable materials, hybrids, digital manufacturing, rapid manufacturing, advanced manufacturing technologies and advanced materials.
Professor Lord Kumar Bhattacharyya, founder and Director of WMG, said: “The governments of the UK and India recognise the importance of close collaboration of both countries in the field of science and technology.
“There is exciting potential for joint research in many fields between us and, in particular, between WMG, Indian private and public research institutions and universities and the Indian private sector. WMG has already been working with very big industries such as Tata and TVS Motors.”
CII Director General Chandrajit Banerjee said: “Indian industries now have a major focus on innovation in order to become path-breakers in their respective fields. There is also a growing need for IPR protection for Indian organisations.
“All economic powers need to respond to the pressing need for new technology to meet the challenges of climate change and environmental damage and India is no different. We also must contribute to the need for a massive improvement in global healthcare provision.
“This link with WMG will give the Indian economy unsurpassed access to new technology in these key areas.”
WMG, an academic department of the University of Warwick, is an internationally renowned research group supporting some of the most advanced research, education and training programmes in the world.
The two organisations will explore areas of cooperation and research collaboration in automotives, experiential engineering, robotics, healthcare, e-security, sustainable materials, hybrids, digital manufacturing, rapid manufacturing, advanced manufacturing technologies and advanced materials.
Professor Lord Kumar Bhattacharyya, founder and Director of WMG, said: “The governments of the UK and India recognise the importance of close collaboration of both countries in the field of science and technology.
“There is exciting potential for joint research in many fields between us and, in particular, between WMG, Indian private and public research institutions and universities and the Indian private sector. WMG has already been working with very big industries such as Tata and TVS Motors.”
CII Director General Chandrajit Banerjee said: “Indian industries now have a major focus on innovation in order to become path-breakers in their respective fields. There is also a growing need for IPR protection for Indian organisations.
“All economic powers need to respond to the pressing need for new technology to meet the challenges of climate change and environmental damage and India is no different. We also must contribute to the need for a massive improvement in global healthcare provision.
“This link with WMG will give the Indian economy unsurpassed access to new technology in these key areas.”
WMG, an academic department of the University of Warwick, is an internationally renowned research group supporting some of the most advanced research, education and training programmes in the world.
Sical Logistics posts Rs.502 mn net profit
Integrated multi modal logistics company Sical Logistics Ltd has closed fiscal 2008 with a turnover of Rs.7.1 billion and a net profit of Rs.502 million as against Rs.10.5 billion turnover and Rs.426.3 million profit posted during fiscal 2007.
While the net profit is up during the period under review as compared to FY07, the dip in the net sales is attributed to the demerger of non logistics business.
According to company officials, the consolidated net sales of Sical's core logistics business was Rs.6.8 billion, up from 6.7 billion a year ago.
Said chairman Ashwin Muthiah: "We successfully executed our strategy of focusing on our core logistics business with the demerger of the non-core business and the separation of the service and infrastructure business. This ensured dedicated management focus and helped us leverage the true potential of each business.
"Our infrastructure projects are progressing smoothly. Over the next two years, most of the special purpose vehicles (SPVs) will commence operations and we envision robust growth and enhanced profitability for the group in the long term.
In May 2007, Sical formed a subsidiary, Sical Infra Assets Ltd, to house the company's asset-heavy, capital-intensive, longer gestation infrastructure-based businesses like the Container Rail Project, the Container terminals at Tuticorin and Chennai in joint venture with PSA Singapore, Sical Iron Ore Terminal at Ennore, Sical Distriparks and Road and Rail terminals at Nagpur.
The short cycle service-oriented businesses and the asset heavy infrastructure businesses of the company were segregated to crystallize the value of investments made by Sical into its SPVs.
In January, Sical received the sanction from the Madras High Court for the de-merger of its non-logistics business into a wholly owned subsidiary, Sicagen India Ltd, with effect from Oct 1, 2006.
The approval marked the successful completion of the de-merger process initiated by Sical in early 2007, and was an important step in the company's restructuring efforts.
The company sold its non-core businesses in FY08, namely the manufacturing facilities and assets of the auto components, refractory, specialty chemicals and flexible shaft divisions.
While the net profit is up during the period under review as compared to FY07, the dip in the net sales is attributed to the demerger of non logistics business.
According to company officials, the consolidated net sales of Sical's core logistics business was Rs.6.8 billion, up from 6.7 billion a year ago.
Said chairman Ashwin Muthiah: "We successfully executed our strategy of focusing on our core logistics business with the demerger of the non-core business and the separation of the service and infrastructure business. This ensured dedicated management focus and helped us leverage the true potential of each business.
"Our infrastructure projects are progressing smoothly. Over the next two years, most of the special purpose vehicles (SPVs) will commence operations and we envision robust growth and enhanced profitability for the group in the long term.
In May 2007, Sical formed a subsidiary, Sical Infra Assets Ltd, to house the company's asset-heavy, capital-intensive, longer gestation infrastructure-based businesses like the Container Rail Project, the Container terminals at Tuticorin and Chennai in joint venture with PSA Singapore, Sical Iron Ore Terminal at Ennore, Sical Distriparks and Road and Rail terminals at Nagpur.
The short cycle service-oriented businesses and the asset heavy infrastructure businesses of the company were segregated to crystallize the value of investments made by Sical into its SPVs.
In January, Sical received the sanction from the Madras High Court for the de-merger of its non-logistics business into a wholly owned subsidiary, Sicagen India Ltd, with effect from Oct 1, 2006.
The approval marked the successful completion of the de-merger process initiated by Sical in early 2007, and was an important step in the company's restructuring efforts.
The company sold its non-core businesses in FY08, namely the manufacturing facilities and assets of the auto components, refractory, specialty chemicals and flexible shaft divisions.
GMR awards toll project contract to Allied Digital
Allied Digital Services Ltd (ADSL), an IT infrastructure management services provider, has bagged a Rs.200 million contract for implementation of toll collection and traffic control solution from leading developer GMR Projects Pvt. Ltd.
GMR has contract of developing four national highway awarded by the National Highway Authority of India (NHAI) that includes the Ambala-Chandigarh Expressway, Jadcherala Expressway, Pochampally Expressway and GMR Ulundurpet Expressway.
ADSL will implement and manage IT infrastructure, intelligent video surveillance, unified communication, automated access traffic controls, and optical scanners for vehicle classification for all the four location, which will be completed by the end of this year.
"Infrastructure companies are showing great interest in IT players to provide infrastructure solutions for their growing business needs," ADSL chairman and managing director Nitin Shah said. "In India, the toll market plays a significant role in this sector and is expected to grow substantially, especially in the light of road projects.”
"The toll market appears very attractive in terms of sheer size and growth potential for the IT industry. It will undoubtedly be a booming business over the next 10 years”, he added.
GMR has contract of developing four national highway awarded by the National Highway Authority of India (NHAI) that includes the Ambala-Chandigarh Expressway, Jadcherala Expressway, Pochampally Expressway and GMR Ulundurpet Expressway.
ADSL will implement and manage IT infrastructure, intelligent video surveillance, unified communication, automated access traffic controls, and optical scanners for vehicle classification for all the four location, which will be completed by the end of this year.
"Infrastructure companies are showing great interest in IT players to provide infrastructure solutions for their growing business needs," ADSL chairman and managing director Nitin Shah said. "In India, the toll market plays a significant role in this sector and is expected to grow substantially, especially in the light of road projects.”
"The toll market appears very attractive in terms of sheer size and growth potential for the IT industry. It will undoubtedly be a booming business over the next 10 years”, he added.
Exide picks up controlling stake in Leadage Alloys India
Battery manufacturing major Exide Industries Ltd has acquired 51 percent stake in Leadage Alloys India Ltd, a lead smelting company near Bangalore, Karnataka, for Rs.340 million.
"Our own lead smelting units will not only give us better self-sufficiency in this critical raw material in the long run. It will also help us have better control over recycling of used batteries that we buy back from the market as part of regulatory framework for storage battery manufacture," T.V. Ramanathan, managing director and CEO of Exide Industries, said here Wednesday.
This is the second lead smelting unit Exide, which manufactures lead acid storage batteries, has picked up controlling stake in after acquiring 100 percent stake of Tandon Metals Ltd in October 2007.
Lead constitutes around 70 percent of the material cost of a battery and its price has remained volatile for the past three years.
For the financial year ended March 31, 2008, the turnover of the company stood at Rs.36.06 billion.
The firm has recently started a nationwide green campaign towards consumer education for the safe disposal of used batteries.
"This investment in our own smelters is not just a backward integration project for Exide. It is part of our commitment to the environment, whereby we will have better control over disposal of used batteries and plastics," said P.K. Kataky, director automotive of Exide Industries.
Exide also has manufacturing facilities in Sri Lanka. It carries out business internationally via its subsidiaries and affiliates in Southeast Asia, Australia and Europe.
"Our own lead smelting units will not only give us better self-sufficiency in this critical raw material in the long run. It will also help us have better control over recycling of used batteries that we buy back from the market as part of regulatory framework for storage battery manufacture," T.V. Ramanathan, managing director and CEO of Exide Industries, said here Wednesday.
This is the second lead smelting unit Exide, which manufactures lead acid storage batteries, has picked up controlling stake in after acquiring 100 percent stake of Tandon Metals Ltd in October 2007.
Lead constitutes around 70 percent of the material cost of a battery and its price has remained volatile for the past three years.
For the financial year ended March 31, 2008, the turnover of the company stood at Rs.36.06 billion.
The firm has recently started a nationwide green campaign towards consumer education for the safe disposal of used batteries.
"This investment in our own smelters is not just a backward integration project for Exide. It is part of our commitment to the environment, whereby we will have better control over disposal of used batteries and plastics," said P.K. Kataky, director automotive of Exide Industries.
Exide also has manufacturing facilities in Sri Lanka. It carries out business internationally via its subsidiaries and affiliates in Southeast Asia, Australia and Europe.
MTNL gets international long distance licence, ISD rates may come down
State-run telecom company Mahanagar Telecom Nigam Ltd (MTNL) received the much-awaited international long distance (ILD) licence, a development that could further lower ISD rates in the country.
The public sector company was issued the licence by the Department of Telecom (DoT) Wednesday.
MTNL is currently routing its international call traffic through other ILD licence holders like VSNL.
The company is gearing up to carry its own traffic in the near future and with this development MTNL's ISD carriage cost would fall significantly.
MTNL, with its operations in Delhi and Mumbai is building an undersea cable project for carrying its own ISD traffic. The project will connect India to Singapore and Malaysia.
The licence is issued on non-exclusive basis, initially for a period of 20 years which is extendable by a period of five years.
The public sector company was issued the licence by the Department of Telecom (DoT) Wednesday.
MTNL is currently routing its international call traffic through other ILD licence holders like VSNL.
The company is gearing up to carry its own traffic in the near future and with this development MTNL's ISD carriage cost would fall significantly.
MTNL, with its operations in Delhi and Mumbai is building an undersea cable project for carrying its own ISD traffic. The project will connect India to Singapore and Malaysia.
The licence is issued on non-exclusive basis, initially for a period of 20 years which is extendable by a period of five years.
UTV's 'The Happening' rakes in 61.5 mn in three days
UTV's foray into the mainstream Hollywood with "The Happening", has paid good dividends to the production house by raking in $31.5 million at the US box-office.
Written and directed by M. Night Shyamalan, the sci-fi thriller that revolves around a family on the run from a natural crisis that presents a large-scale threat to humanity, was released June 13.
Quoting the box-office collection figure from the Hollywood trade magazine "Variety", UTV stated that "The Happening", its first major collaborative venture with 20th Century Fox, has performed well at the world box-office. It has earned a revenue of $31.5 million at the US box-office in the first three days of its release.
"The Happening" was made on a budget of $55 million.
This, despite the fact that the movie did not receive a very favourable review from the US media. In addition, it has garnered $30 million from the rest of the world from 5,600 prints.
In Britain, with a collection of $3.4 million from 389 prints in three days, the film has been ranked third at the box office chart.
UTV's earlier $8 million co-production with Fox, "Namesake" had grossed $18 million at the worldwide box-office and almost an equal amount from the home video and TV syndication.
Considering its theatrical performance so far, "The Happening" may be expected to do equally well in the home video market and also on TV syndication.
Written and directed by M. Night Shyamalan, the sci-fi thriller that revolves around a family on the run from a natural crisis that presents a large-scale threat to humanity, was released June 13.
Quoting the box-office collection figure from the Hollywood trade magazine "Variety", UTV stated that "The Happening", its first major collaborative venture with 20th Century Fox, has performed well at the world box-office. It has earned a revenue of $31.5 million at the US box-office in the first three days of its release.
"The Happening" was made on a budget of $55 million.
This, despite the fact that the movie did not receive a very favourable review from the US media. In addition, it has garnered $30 million from the rest of the world from 5,600 prints.
In Britain, with a collection of $3.4 million from 389 prints in three days, the film has been ranked third at the box office chart.
UTV's earlier $8 million co-production with Fox, "Namesake" had grossed $18 million at the worldwide box-office and almost an equal amount from the home video and TV syndication.
Considering its theatrical performance so far, "The Happening" may be expected to do equally well in the home video market and also on TV syndication.
Tuesday, June 17, 2008
Air Canada axes 2,000 jobs, flights to cut costs
 Air Canada Tuesday announced it would axe 2,000 jobs this year to meet the rising fuel costs.
Air Canada Tuesday announced it would axe 2,000 jobs this year to meet the rising fuel costs.Canada's premier airline was also scaling back flights on some long-haul routes as well as autumn and winter services in North America.
In a statement, Air Canada president and CEO Montie Brewer said: "The loss of jobs is painful in view of our employees' hard work in bringing the airline back to profitability over the past four years.
"I regret having to take these actions but they are necessary to remain competitive going forward. Air Canada, like most global airlines, needs to adapt its business and reduce flying that has become unprofitable in the current fuel environment."
He warned that if fuel prices remain at current levels, "we can anticipate further capacity reductions."
Voted the best airline in North America in the 2007 Skytrax World Airline Awards, Air Canada has been struggling to cut costs as each extra dollar it pays on fuel imposes an additional annual burden of $26 million on it.
The airline, which spends 30 percent of its budget on fuel, said it will be forced to shell out an additional $1 billion on it .
The long-haul routes on the chop include the Toronto-Rome and Vancouver-Osaka non-stop flights, thus reducing its international services by seven percent.
From the last quarter of this year and the first quarter of 2009, the airline will scale back domestic flights by two percent and trans-border (US) flights by 13 percent.
Tech Mahindra Enters into Strategic Internet Protocol TV (IPTV) Global Alliance
Tech Mahindra, India's sixth largest software exporter and largest solution provider focused on global telecom industry, has entered into a strategic global alliance with Microsoft Corp. to address its System Integration (SI) requirements for deployments of the award-winning Microsoft Mediaroom Internet Protocol Television (IPTV) and multi-media software platform. Through the alliance, Tech Mahindra's solution will provide deployment and integration services for new and existing Microsoft Mediaroom broadband service provider customers to deploy IPTV services, which are expected to gain momentum in the coming years.
The alliance will further empower Tech Mahindra in its quest to implement best-performing IPTV solutions and support cutting-edge technologies in interactive digital television. It will also help Tech Mahindra strengthen its IPTV SI capabilities and give both companies an opportunity to expand into new markets.
The alliance, which builds on Tech Mahindra's leadership in integrating end-to-end multimedia solutions and Microsoft's proven success in bringing connected TV ser-vices to market, will give service providers another way to accelerate and differenti-ate their TV offerings. Microsoft Mediaroom, which enables broadband service pro-viders to deliver new connected TV and entertainment experiences to consumers, is already being deployed or trialed by over 20 service providers worldwide.
According to Mr. CP Gurnani, President Tech Mahindra: "Tech Mahindra through its sustained investments in R&D, has developed strong IPTV capabilities across several areas that include: system integration capabilities, product engineering, infrastruc-ture deployment and specific BSS/OSS capabilities. Tech Mahindra is engaged with a number of incumbent and emerging service providers in the IPTV domain and Triple Play around the world. We are very excited about this alliance with Microsoft as the relationship presents a tremendous opportunity for both organizations to strengthen our leadership positions in IPTV "
"We are committed to teaming with best-in-class technology partners to deliver the best connected TV services using Microsoft Mediaroom," said Joe Seidel, director of global partner ecosystems for Microsoft Mediaroom. "Tech Mahindra was a natural choice for us given its experience in digital video solutions and services. Adoption of our platform continues to accelerate, and we are excited the company is joining our ecosystem at this crucial inflection point."
About Tech Mahindra:
Tech Mahindra is a leading provider of solutions and services to the telecommunications industry, majority stake owned by Mahindra & Mahindra, in partnership with BT Plc. With total revenues of USD 943 million in the year ended March 31, 2008, Tech Mahindra is India's 6th largest software exporter, and serves telecom service providers, equipment manufacturers, software vendors and systems integrators. Tech Mahindra solutions enable clients to maximize returns on IT investment by achieving fast time to market reduced total cost of ownership and high customer satisfaction. Tech Mahindra achieves this through its domain and process expertise, distinctive IT skills, research and development, proven innovative delivery models and approach to off shoring. Assessed at SEI-CMMi Level 5 and PCMM Level 5, Tech Mahindra's track record for value-delivery is supported by over 23,000 professionals who provide a unique blend of culture, domain expertise and in-depth technology skill-sets. Its development centres are ISO 9001:2000 & BS7799 certified. Tech Mahindra has prin-cipal offices in the UK, United States, Germany, UAE, Egypt, Singapore, India, Thai-land, Malaysia and Indonesia.
For more information visit: www.techmahindra.com
The alliance will further empower Tech Mahindra in its quest to implement best-performing IPTV solutions and support cutting-edge technologies in interactive digital television. It will also help Tech Mahindra strengthen its IPTV SI capabilities and give both companies an opportunity to expand into new markets.
The alliance, which builds on Tech Mahindra's leadership in integrating end-to-end multimedia solutions and Microsoft's proven success in bringing connected TV ser-vices to market, will give service providers another way to accelerate and differenti-ate their TV offerings. Microsoft Mediaroom, which enables broadband service pro-viders to deliver new connected TV and entertainment experiences to consumers, is already being deployed or trialed by over 20 service providers worldwide.
According to Mr. CP Gurnani, President Tech Mahindra: "Tech Mahindra through its sustained investments in R&D, has developed strong IPTV capabilities across several areas that include: system integration capabilities, product engineering, infrastruc-ture deployment and specific BSS/OSS capabilities. Tech Mahindra is engaged with a number of incumbent and emerging service providers in the IPTV domain and Triple Play around the world. We are very excited about this alliance with Microsoft as the relationship presents a tremendous opportunity for both organizations to strengthen our leadership positions in IPTV "
"We are committed to teaming with best-in-class technology partners to deliver the best connected TV services using Microsoft Mediaroom," said Joe Seidel, director of global partner ecosystems for Microsoft Mediaroom. "Tech Mahindra was a natural choice for us given its experience in digital video solutions and services. Adoption of our platform continues to accelerate, and we are excited the company is joining our ecosystem at this crucial inflection point."
About Tech Mahindra:
Tech Mahindra is a leading provider of solutions and services to the telecommunications industry, majority stake owned by Mahindra & Mahindra, in partnership with BT Plc. With total revenues of USD 943 million in the year ended March 31, 2008, Tech Mahindra is India's 6th largest software exporter, and serves telecom service providers, equipment manufacturers, software vendors and systems integrators. Tech Mahindra solutions enable clients to maximize returns on IT investment by achieving fast time to market reduced total cost of ownership and high customer satisfaction. Tech Mahindra achieves this through its domain and process expertise, distinctive IT skills, research and development, proven innovative delivery models and approach to off shoring. Assessed at SEI-CMMi Level 5 and PCMM Level 5, Tech Mahindra's track record for value-delivery is supported by over 23,000 professionals who provide a unique blend of culture, domain expertise and in-depth technology skill-sets. Its development centres are ISO 9001:2000 & BS7799 certified. Tech Mahindra has prin-cipal offices in the UK, United States, Germany, UAE, Egypt, Singapore, India, Thai-land, Malaysia and Indonesia.
For more information visit: www.techmahindra.com
Sunday, June 15, 2008
Press Release about icontrol: The IPTV
Read my first post to know more about icontrol.
Aksh Optifibre, a public listed company, the second largest company and a large-scale manufacturer of cable and optical fibre. It was incorporated in the year 1986 as a private limited company and was converted into a public limited company on 13th March 1994. Aksh has two plants at Bhiwadi, for manufacturing optical fibre cables and fibre, and another at Reengus, Rajasthan. Aksh has recently developed a new cost-effective concept called “Fibre-to-Home”, through which they expect to revolutionise the IT and telecom industry completely. It’s a concept that would bring wireless Voice, Audio, Video and Internet direct to home. The company is planning a Rs 200-crore expansion for the recent future. The company is betting big in the emerging IPTV (Internet Protocol Television) segment in India.
AKSH Optifibre Ltd. is the first company to launch icontrol brand in Delhi and Mumbai on to promote IPTV in Indiauin association with MTNL. Aksh has recently signed an agreement with BSNL also to provide IPTV services in 20 cities in North India , the states of Jammu & Kashmir, Punjab, Haryana, Rajasthan and UP (West) etc The company plans to add about 5 lakh subscribers for our IPTV services in Delhi and Mumbai in next 3 years. Aksh is investing close to $37 million in its services business on IPTV and VoIP platforms.
icontrol service provided by Aksh in association with MTNL is a IPTV based television connection that is to consumers to watch the TV programs they wish to view at their own convenient time. icontrol offers 120 channels, 200 movies on demand with rewind, fast forward and pause option. icontrol requires NO COMPUTER, NO INTERNET, NO BROADBAND, NO DVD PLAYER.
Speaking at the launch of icontrol, Dr Choudhari, MD, Aksh Optifibre said ” All icontrol subscribers can replay Live channels and view your favorite program time & again through Time shift TV. icontrol will let you do interactive gaming, messaging on television which are not available on cable and DTH. There is a misconception that for viewing IPTV, broadband and PC is necessary. You need not have any computers, special hardware or technical knowledge. icontrol will do to India, what PC did to US”
The I in icontrol stands for the Interactivity factor prevalent in IPTV. icontrol is revolutionary as all it needs is a TV, broadband is no longer required. IPTV in colloquial term stands for “Internet Protocol TV” simply means “Interactive Personalized TV”.
Aksh’s icontrol is designed so as to enable consumers to control the television, to convert the idiot box into a utility box and in establishing two-way communication for the consumer. This means you request for a program and you are delivered the same. The launch strategy for icontrol brand is to position “TV IS SERVANT”. icontrol promises it’s consumers, control over TV viewing. The IPTV/VOIP business is slated to grow in an exponential manner over the next few years. And it is estimated that in 3 years time, this division at Aksh alone can reach a turnover of around Rs 3500 crores.MTNL has 1.52 lakh broadband subscribers in Delhi and 1.83 lakh in Mumbai. MTNL will offer this service to its broadband subscribers using its existing broadband network. “Consumers have come to expect sterling services that are both innovative and affordable from MTNL,” said Shri RSP Sinha, chairman and managing director at MTNL. “We take the lead, yet again, in ushering in a true revolution in home entertainment with the country’s first IPTV service. IPTV provides an interactive viewing experience and allows for multitude of exciting revenue generating applications to be deployed. Our existing presence in more than four million homes in New Delhi and Mumbai through our broadband service will give MTNL a tremendous headstart in changing the way people watch television.”Aksh Optifiber aims to provide IPTV service to 450,000 subscribers in Mumbai and Delhi, for which it utilizes MTNL’s existing broadband network as the content delivery backbone and enable users to get services such as video on demand, broadband, TV channels, time-shifted TV, and video conferencing.
The service, which will utilize MTNL’s existing broadband network as the content delivery backbone, will consist of traditional broadcast television, video- and music-on-demand, videoconferencing capabilities and time-shifted TV, allowing users to access television content on any channel up to one week in the past.
About the Owners: Choudhari and Navani family originally promoted AOL with Dr. Kailash S. Choudhari as the sole promoter. Dr. Choudhari is a technocrat with over 15 years of experience in the cable industry. He holds a stake of 19% in AOL. AOL originally used to manufacture and export PVC and PE insulated speciality cables before diversifying into optical fibre cables.
Aksh Optifibre, a public listed company, the second largest company and a large-scale manufacturer of cable and optical fibre. It was incorporated in the year 1986 as a private limited company and was converted into a public limited company on 13th March 1994. Aksh has two plants at Bhiwadi, for manufacturing optical fibre cables and fibre, and another at Reengus, Rajasthan. Aksh has recently developed a new cost-effective concept called “Fibre-to-Home”, through which they expect to revolutionise the IT and telecom industry completely. It’s a concept that would bring wireless Voice, Audio, Video and Internet direct to home. The company is planning a Rs 200-crore expansion for the recent future. The company is betting big in the emerging IPTV (Internet Protocol Television) segment in India.
AKSH Optifibre Ltd. is the first company to launch icontrol brand in Delhi and Mumbai on to promote IPTV in Indiauin association with MTNL. Aksh has recently signed an agreement with BSNL also to provide IPTV services in 20 cities in North India , the states of Jammu & Kashmir, Punjab, Haryana, Rajasthan and UP (West) etc The company plans to add about 5 lakh subscribers for our IPTV services in Delhi and Mumbai in next 3 years. Aksh is investing close to $37 million in its services business on IPTV and VoIP platforms.
icontrol service provided by Aksh in association with MTNL is a IPTV based television connection that is to consumers to watch the TV programs they wish to view at their own convenient time. icontrol offers 120 channels, 200 movies on demand with rewind, fast forward and pause option. icontrol requires NO COMPUTER, NO INTERNET, NO BROADBAND, NO DVD PLAYER.
Speaking at the launch of icontrol, Dr Choudhari, MD, Aksh Optifibre said ” All icontrol subscribers can replay Live channels and view your favorite program time & again through Time shift TV. icontrol will let you do interactive gaming, messaging on television which are not available on cable and DTH. There is a misconception that for viewing IPTV, broadband and PC is necessary. You need not have any computers, special hardware or technical knowledge. icontrol will do to India, what PC did to US”
The I in icontrol stands for the Interactivity factor prevalent in IPTV. icontrol is revolutionary as all it needs is a TV, broadband is no longer required. IPTV in colloquial term stands for “Internet Protocol TV” simply means “Interactive Personalized TV”.
Aksh’s icontrol is designed so as to enable consumers to control the television, to convert the idiot box into a utility box and in establishing two-way communication for the consumer. This means you request for a program and you are delivered the same. The launch strategy for icontrol brand is to position “TV IS SERVANT”. icontrol promises it’s consumers, control over TV viewing. The IPTV/VOIP business is slated to grow in an exponential manner over the next few years. And it is estimated that in 3 years time, this division at Aksh alone can reach a turnover of around Rs 3500 crores.MTNL has 1.52 lakh broadband subscribers in Delhi and 1.83 lakh in Mumbai. MTNL will offer this service to its broadband subscribers using its existing broadband network. “Consumers have come to expect sterling services that are both innovative and affordable from MTNL,” said Shri RSP Sinha, chairman and managing director at MTNL. “We take the lead, yet again, in ushering in a true revolution in home entertainment with the country’s first IPTV service. IPTV provides an interactive viewing experience and allows for multitude of exciting revenue generating applications to be deployed. Our existing presence in more than four million homes in New Delhi and Mumbai through our broadband service will give MTNL a tremendous headstart in changing the way people watch television.”Aksh Optifiber aims to provide IPTV service to 450,000 subscribers in Mumbai and Delhi, for which it utilizes MTNL’s existing broadband network as the content delivery backbone and enable users to get services such as video on demand, broadband, TV channels, time-shifted TV, and video conferencing.
The service, which will utilize MTNL’s existing broadband network as the content delivery backbone, will consist of traditional broadcast television, video- and music-on-demand, videoconferencing capabilities and time-shifted TV, allowing users to access television content on any channel up to one week in the past.
About the Owners: Choudhari and Navani family originally promoted AOL with Dr. Kailash S. Choudhari as the sole promoter. Dr. Choudhari is a technocrat with over 15 years of experience in the cable industry. He holds a stake of 19% in AOL. AOL originally used to manufacture and export PVC and PE insulated speciality cables before diversifying into optical fibre cables.
MTNL and Aksh launch icontrol in Delhi and Mumbai; Know basics of icontrol
Ok. So Lets try to find out what is really "icontrol" is...
Firstly lets talk about IPTV:
IPTV (Internet Protocol Television) is a system where a digital television service is delivered using Internet Protocol over a network infrastructure, which may include delivery by a broadband connection. A general definition of IPTV is television content that, instead of being delivered through traditional broadcast and cable formats, is received by the viewer through the technologies used for computer networks.
icontrol advt says that you do not need any broadband service or telephone to use this service, Thats right, icontrol will use a optical fibre cable to transfer television content to you TV. Ad it is totally based on computer netwokrs so you will have an added advantage to control what you are going to watch.
icontrol tarrifs
Delhi :
* Free Setup Box against fully refundable security deposit of Rs. 999.
* Pay Rs.199 per month to get icontrol service through MTNL.
* No Telephone , No computer, No DVD Player, No Internet / Broadband required for using icontrol
Mumbai :
* icontrol currently for MTNL broadband subscribers.
* Free Setup box against fully refundable security deposit of Rs. 999.
* Pay Rs. 100 per month to get icontrol service through MTNL.
* No Telephone , No computer, No DVD Player, No Internet / Broadband required for using icontrol
But since it has just been launched, I too was not able to go through the service, but i hope this change the viewing experience of Delhi TV viewers.
Saturday, June 14, 2008
HTC P3350 in India now, Price: Rs 18490
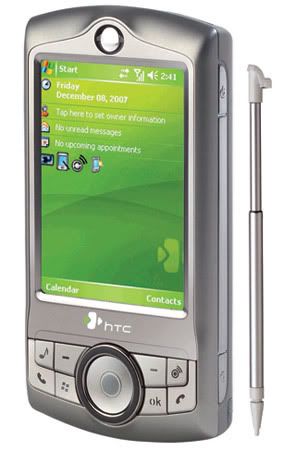 HTC has launched the P3350 phone in the Indian market. The latest HTC phone offers business features packed in a compact, slim shell. Besides, entertainment of the users is ensured through music capabilities.
HTC has launched the P3350 phone in the Indian market. The latest HTC phone offers business features packed in a compact, slim shell. Besides, entertainment of the users is ensured through music capabilities.The HTC P3350 features in-built FM radio and is further capable of downloading music and audio-visual content. WLAN access for fast speed downloading is another key feature of the newly released phone.
Apart from WLAN, the P3350 features other connectivity options such as quadband, GPRS, EDGE and Bluetooth v2.0. The phone runs on the Windows Mobile 6.0 platform.
The P3350 does not only have smart functions but also looks stylish. The first noticeable feature of the phone is its 360-degree scroll wheel and 8-way touch pointer, which is a new innovative interface design.
Announcing the availability of the handset, Ajay Sharma, Country Manager, HTC India, said, “The launch of the HTC P3350 is line with our efforts of offering a device that combines and supports data capabilities along with a platform to provide and support music and entertainment experience for users while on the move. We are positive that this device will be a big hit among music enthusiasts.”
Some of the other features the HTC P3350 phone are:
# A 2.8 inch QVGA display screen
# A 2.0 megapixel CMOS camera
# Microsoft Office programs like Outlook Mobile, Word Mobile, Excel Mobile, PowerPoint Mobile, IE Mobile, DirectPush and Windows Media Player 10 Mobile
# Adobe Reader (PDF), Activesync, Comm Manager, Internet sharing, Network Wizard, Audio Manager and Audio Booster
The HTC P3350 phone is available in the Indian market at the price of Rs.18,490.
About HTC:
HTC Corp, (TAIEX: 2498) produces powerful handsets that continually push the boundaries of innovation to provide true mobile freedom.
Founded in 1997 by Cher Wang, Chairwoman, HT Cho, Director of the Board & Chairman of HTC Foundation, and Peter Chou, President and CEO, HTC made its name as the company behind many of the most popular operator-branded devices on the market. It has established unique partnerships with key mobile brands, including the leading five operators in Europe, the top four in the US, and many fast-growing Asian operators. It has also brought products to market with industry leading OEM partners and, since June 2006, under its own HTC brand.
HTC is one of the fastest-growing companies in the mobile sector and has achieved remarkable recognition over the past couple of years. Business Week ranked HTC as the second best performing technology company in Asia in 2007 as well as giving the company the number 3 spot in its Global listing in 2006.
Since launching its own brand 18 months ago the company has introduced dozens of HTC-branded products around the world.
Other Products & Innovation
HTC is known for its innovation. It is constantly broadening the range of devices it offers – introducing devices to support specific applications and new form factors that meet the increasingly diverse needs of its customers and partners.
HTC's product portfolio offers easy-to-use solutions that embrace the full range of mobile multimedia resources, wireless anytime and Internet on the go.
It has a rich heritage of device 'firsts':
First color palm-size PC (1999)
First Microsoft Pocket PC (2000)
First Microsoft wireless Pocket PC (2002)
First Microsoft powered Smartphone (2002)
First Microsoft Smart Music Phone (2004)
Large 2.8" TFT touch-screen LCD display
First Microsoft 3G Phone (2005)
First Microsoft Windows Mobile 5.0 Platform Phone (2005)
First tri-band UMTS 3G device on the Microsoft Windows Mobile platform (2006)
First Microsoft Windows 5.0 Smartphone (2006)
First Tri-band UMTS PDA
First intuitive touch screen to allow finger tip navigation (June 2007)
In early 2006, HTC launched a powerful new device with a groundbreaking form factor: the HTC Advantage. The HTC Advantage is the world’s most powerful office, boasting a 5" screen and full detachable QWERTY keyboard. This was followed in early 2007 with the introduction of the HTC Shift. Equipped with Windows Vista this device includes a brilliant 7-inch widescreen touch display and a 40 gigabyte hard drive.
HTC launched the HTC Touch™ in June 2007 as the result of extensive R&D and the conviction that fingertip control would enable more intuitive navigation. The groundbreaking HTC Touch™ is equipped with TouchFLO™ so that consumers just sweep their finger across the screen to get access to the most commonly used content, contacts and features in a simple finger flick.
HTC aims to continually develop smart new devices that empower users on the go, providing more freedom in the way they live their lives.
Wednesday, June 11, 2008
Ranbaxy-Daiichi marriage: Implications for Indian pharma
Japan's second-largest pharma company, Daiichi Sankyo will buy out the entire promoter stake of 35% in Ranbaxy Laboratories at Rs 737 per share. The deal amounts to about USD 2.7-3.7 billion. The Japanese company is eyeing 51% stake in Ranbaxy.
What does this deal mean for both Ranbaxy and Daiichi?
According to D S Brar, who actually steered the company for 20 years and made it into the first billion dollar company from the Indian pharma space, said, “It is probably great news for Ranbaxy shareholders to have this kind of a premium. Many of them will not have expected this kind of a thing.”
Even future upsides are probably factored into this particular deal for Ranbaxy. For Daiichi, it makes sense to have a large research and manufacturing background that Ranbaxy has.
Daiichi makes sense for Ranbaxy because it has got 2-3 big research products to club with the Indian pharma major. It will attack the regulated market with a generic play. Also, its R&D pipeline products will make sense for Daiichi. So, it makes sense for both companies and shareholders.
But what does this marriage mean for Indian pharma companies?
Dr Anji Reddy of Dr Reddy’s Laboratories had a different view. He said, "I can’t imagine selling my company at whatever value. My research pipeline is robust and I believe my company will grow well.”
N Prasasd, who sold his Matrix stake to Mylan Laboratories, said, “Consolidation will be important for the Indian drug industry as a whole to have a global play.”
On whether an open offer might happen:
This is completely taken as a safeguard. We have not been able to get any lead on this. This probably has been cleared by Malvinder Singh and Atul Sobti his Deputy. We have not got any clues from any investment bankers. All of them are equally listening about this entire deal.
What does this deal mean for both Ranbaxy and Daiichi?
According to D S Brar, who actually steered the company for 20 years and made it into the first billion dollar company from the Indian pharma space, said, “It is probably great news for Ranbaxy shareholders to have this kind of a premium. Many of them will not have expected this kind of a thing.”
Even future upsides are probably factored into this particular deal for Ranbaxy. For Daiichi, it makes sense to have a large research and manufacturing background that Ranbaxy has.
Daiichi makes sense for Ranbaxy because it has got 2-3 big research products to club with the Indian pharma major. It will attack the regulated market with a generic play. Also, its R&D pipeline products will make sense for Daiichi. So, it makes sense for both companies and shareholders.
But what does this marriage mean for Indian pharma companies?
Dr Anji Reddy of Dr Reddy’s Laboratories had a different view. He said, "I can’t imagine selling my company at whatever value. My research pipeline is robust and I believe my company will grow well.”
N Prasasd, who sold his Matrix stake to Mylan Laboratories, said, “Consolidation will be important for the Indian drug industry as a whole to have a global play.”
On whether an open offer might happen:
This is completely taken as a safeguard. We have not been able to get any lead on this. This probably has been cleared by Malvinder Singh and Atul Sobti his Deputy. We have not got any clues from any investment bankers. All of them are equally listening about this entire deal.
Ranbaxy promoters to sell stake to Japan's Daiichi
 Daiichi to get 50.1% stake in Ranbaxy
Daiichi to get 50.1% stake in RanbaxyRanbaxy valued at $8.8 billion with Daiichi paying Rs737 per share at 70% premium to market cap
In a major development, Japanese pharmaceutical company Daiichi Sankyo is set to procure the complete promoter’s stake of 34.82% in one of India’s biggest pharmaceutical company Ranbaxy Laboratories at Rs737 per share. $440 million of FCB will also be converted into equity, present Ranbaxy CEO Malvinder Mohan Singh informed at the company’s press meet to announce the same. The conversion is expected to happen at Rs540- 550 per share. In all, the Japanese Company will have 50.1% stake in the company, said Singh.
With the present valuations, Ranbaxy is now valued at $8.5 billion on diluted basis. It will become a debt free company after the deal. It will also remain listed on the Indian bourses. Religare Capital Advisors were the managers of the deal.
Daichi will acquire the stake through a tender offer and has valued Ranbaxy at $8.8 billion — a 70% premium over existing market capitalisation.
As per the arrangement, Malvinder Mohan Singh will continue to remain the CEO and Managing Director.There will be no change in the company’s present management. Singh will also be a part of the Daiichi global management team and global board.
“Additionally, upon closing he would assume the position of Chairman of the Board,” an official release said.
The value of the deal is expected to be above $3 billion.
Ranbaxy shares were trading up at Rs585.10 at 12pm
At present (up to 31, March 2008), FII and institutional investors hold close to 41.28%. The general public holds around 18.53% in Ranbaxy.
For the full year ended December 2007, the company reported an operating income of Rs4,293.02 crore with net income of Rs617.72 crore.
Daiichi Sankyo is the joint holding company established in 2005 by Sankyo Co and and Daiichi Pharmaceuticals, both based out of Japan.
History of Ranbaxy:
1961
- The Company was Incorporated on 16th June, 1961 at Delhi. The Company Manufacture drugs, medicines, cosmetics and chemical products. The company also markets a wide range of products including a number of life saving antibiotics.
1973
- The shareholders of the Company offered for sale to the public during October simultaneously with the public issue of shares 63,535 equity shares of Rs 10 each of the Company at par.
1977
- The Company had set up a joint venture company with local participation in Nigeria. The equipments to be supplied against the Companmy's contribution to the equity capital were ordered. These were shipped during 1978 and were received and installed in Nigeria. Production commenced in early 1980.
1984
- Ranbaxy Investments Ltd., Medicare Investments Ltd., and Cheminvest Ltd., became subsidiaries of the Company.
1985
- Approval of the Malaysian Government was received for a joint venture project. The project commenced production in July 1987.
- In October the Company made a rights issue of 1,00,000 equity shares of Rs 10 each linked to 15,00,000-15% secured redeemable non-convertible debentures of Rs 100 each, both at par on the following basis:
- (i) 9,09,000 equity shares tied to 13,63,500-15% debentures to the existing equity shareholders and holders of the convertible parts of 13.5% secured debentures in the ratio 27 debentures and 18 equity shares for every 50 equity shares or 10 convertible parts of the 13.5% debentures held,
- (ii) 71,000 equity shares tied to 1,06,500 debentures as preferential allotment to equitable basis to employees, and
- (iii) 20,000 equity shares tied to 30,000 debentures to business associates. The issue was oversubscribed and the Company allotted a further 2,50,000 equity shares tied to 3,75,000 bedentures to retain oversubscription.
- Ranbaxy Drugs Ltd., became a subsidiary of the Company. Montari Agro Chem Investment Ltd., Montari Chemicare Investments Ltd., Chahel Investment & Trading Co., Ranpharm Investment Ltd., Raicure Investment Ltd., & Vidyut Inv. are subsidiaries of the Company.
1986
- 9.8% Pref. shares redeemed. 39,510 equity shares issued in conversion of 13.5% debentures.
1987
- An antibiotic plant at Toansa, Dist. Hoshiarpur (Punjab) and the Nalidixic Acid plant as SAS Nagar (Punjab) were commissioned.
- 7,000-11% Pref. shares redeemed. 45,680 equity shares issued in conversion of 13.5% debentures. (Final conversion).
1988
- A new broad spectrum oral fluoroquinolone of loxacin under the brand name "Zanocin" was launched in the market in April 1990.
- The Company offered 16,64,775-12.5% secured fully convertible debentures of Rs 100 each for cash at par aggregating to Rs 16,64,77,500. Of these, 15,85,500 debentures were offered to the shareholders on rights basis in the proportion 30 debentures: 100 equity shares (all were taken up). The balance 79,275 debentures were offered to the employees (including Indian working Directors) (all were taken up). Additional 2,49,716 debentures were allotted to retain oversubscription (2,37,825 debentures to the shareholders and 11,891 debentures to the employees).
- Each debenture was converted into 2 equity shares of Rs 10 each at a premium of Rs 40 per share on 31.3.1989. Accordingly, 38,28,982 equity shares of Rs 10 each were issued. Subject to necessary approvals being obtained, the Company proposed to issue 13,67,097-12.5% secured redeemable partly convertible debentures of Rs 300 each on rights basis to the existing equity shareholders in the ratio of 9 debentures for every 100 equity shares held. Simultaneously, the Company proposed to offer, 68,355 debentures to the employees/workers of the Company.
- As per the proposed terms of issue, part-A of Rs 200 of each debenture will be converted into four equity shares of Rs 10 each at a premium of Rs 40 per share at the end of 6 months from the date of allotment. The non-convertible part-B of each debenture will be redeemed at part at the end of 7th year from the date of allotment.
1990
- A new plant was set up at Sahibzada Ajit Singh Nagar for the production was commissioned. A bulk facility to produce newer fluoroquinolones was also being set up at Dewas.
- On 1st June, the scheme of arrangement for spinning-off the pharmaceutical plant at Okhla, New Delhi of the Company to Pharmax Corporation Ltd. was approved by the Honourable high court of Punjab & Haryana.
- Tata Hotels Ltd., is a subsidiary of the Company with a holding of 7,500 equity shares of Rs 10 each out of 7,600 equity shares issued. Since 1990, Tata Hotels Ltd. ceased to be a subsidiary of the company.
1991
- The Company proposed to undertake backward integration into fermentation based products such as Penicillin and Cephalosporin-C. These were to be implemented at Paonta Sahib in Himachal Pradesh, a new complex being developed for bulk drugs.
- 2,250 Pref. shares redeemed on 3.9.91. 60,75,988 bonus equity shares issued in prop. 2:3.
1992
- The Company offered 13,67,097-12.5% partly convertible debentures of Rs 300 each on Rights basis in the proportion of 9 debentures: 100 equity shares held. All were taken up. Additional 2,05,065 debentures were allotted to retain oversubscription.
- 68,355 debentures of Rs 300 each were also issued to the employees' on an equitable basis. (All were taken up). Additional 10,253 debentures were allotted to retain oversubscription.
- Part A of Rs 200 of the face value of each debenture was to be compulsorily converted into 4 equity shares of Rs 10 each at a premium of Rs 40 per share at the end of six months from the date of allotment of debentures.
- Part B of Rs 100 of the face value of each debenture was to be redeemed at par at the end of 7 years from the date of allotment of debentures.
- RLL has three successful overseas joint ventures in Nigeria, Malaysia and Thailand. A joint venture recently incorporated in India with Eli Lilly -- a leading original research company in pharmaceuticals
1993
- During September the Company offered 24,51,719-12% fully convertible debentures of Rs 300 each on Rights basis in the proportion 15 debs: 200 equity shares held (all were taken up) (18611 debs. were kept in abeyance).
- Another 1,22,586-12% debentures were offered to the employees as an equitable basis (all were taken up).
- Part A of Rs 125 of each debenture was to be converted into one equity share of Rs 10 each at a premium of Rs 15 per share on the date of allotment. Accordingly 25,55,694 equity shares were allotted on 5.12.93.
- Part B of Rs 175 of each debenture was to be converted into one equity share of Rs 10 each at a premium of Rs 165 per share on the expiry of 12 months from the date of allotment. Accordingly 25,55,694 equity shares were allotted on 6.12.1994.
- The Company also offered 40,86,197-15% Non-Convertible debentures with warrants attached to it on Rights basis in the proportion 25 NCDS with warrants 8,200 equity shares held (all were taken up).
- Another 2,04,310-15% NCDS were also offered to the employees on an equitable basis (all were taken up). These would be redeemed in three instalments of Rs 66, Rs 67 and Rs 67 each at the end of 6th, 7th & 8th year respectively from the date of allotment debentures.
- Each NCP carries a warrant entitling the holder to apply for one equity share of Rs 10 each at a premium of Rs 115 per share at one or more instalment as may be decided by the Board during the period between 36th months and 60th months from the date of allotment of NCDS at half yearly intervals.
- The Company also offered 85,000-12% Full Convertible debentures with warrants of Rs 300 each for cash at par to Specified Entities of the Management Group. Part A of debenture would be converted into one equity share of Rs 10 each at a premium of Rs 115 per share on the date of allotment. Part B of Rs 175 of each debenture was to be converted into one equity share of Rs 10 at a premium of Rs 165 per share. On December 6th 1994, 26,40,694 equity shares of Rs 10 ech were allotted as per the terms of fully convertible debentures issued in December 1993, Part B of Rs 175 each.
- Each debenture has a warrant attached to the entitling the holder to apply for 25 equity shares of Rs 10 each for cash at a premium of Rs 165 per share in one or more instalment as decided by the Board, during the period between expiry of 12th month and 60th months from the date of allotment of debentures.
- On 5th December, 32,81,154 equity shares were issued to shareholders of erstwhile Crosslands Research Laboratories Ltd. on its amalgamation with the Company.
- It went public in Oct.'93 to part-finance manufacturing facilities of bulk fluoroquinolones at Dewas, MP; and dosage forms at Paonta Sahib, Himachal Pradesh. For easier access to the European markets
1994
- The Expansion and Modernisation plant for manufacture of Ranitidine and Amoxycillin and a pilot plant for R&D at Toansa were commissioned. A new plant for manufacture of pharmaceutical dosage forms at Paonta Sahib was completed.
- The company allotted one hundred thousand warrants without any face value to entities of the management group on 28th July. Each of such warrants entitles the holder to subscribe to fifty equity shares of Rs 10 each at a premium of Rs 390 per share within a period of 18 months from the date of allotment.
- The Company offered 51,61,290 GDSs representing 51,61,290 equity share of Rs 10 each at a price of US $19.375 equivalent to 604.89 (at the rate of US $1.00 = 31.22) per GDS on 29th June.
1995
- A Global Alliance Agreement was signed with Eli Lilly and the Company, for marketing of pharmaceutical products in U.S.A. and other countries. For the purpose of Indian joint venture, a company under the name Ranbaxy Lilly company was incorporated.
- A join venture in U.S.A. for marketing of products from the Indian joint venture as also select Lilly and Ranbaxy products was proposed. For the purpose, a company under the name "Lilly Ranbaxy Pharmaceuticals LLC" was incorporated in the state of Indiana, U.S.A.
1996
- RLL bought a drug firm in Ireland in Jan.'96. In 1996, it acquired six leading brands from Gufic. Croslands Research Laboratories, a leading manufacturer of dermatological pharmaceutical formulations has been merged with RLL. In Oct.'98 sold off the Glat (Global Alliances and Technologies) division of Croslands to French pharma major Galderma.
1997
- The name of Ranmax Laboratories (Nigeria) Ltd., was changed to Ranbaxy (Nigeria) Ltd.
- 1,50,000 equity shares of Rs 10 each (prem. Rs 630 per share) allotted on 12th June, to Ranbaxy Employees Welfare Society on exercise of rights attached with warrants allotted to the society. 2,23,958 equity shares of Rs 10 each (prem. Rs 115 per share) allotted on 1.8.1997 on exercise of right attached with warrants allotted along with NCDs of Rs 200 each on rights basis. 6,56,423 equity shares of Rs 10 each (prem. Rs 115 per share) allotted on 1st February 1998 on exercise of right attached with warrants allotted along with NCDs of Rs 200 each on rights basis - The Company has licence arrangements with M/s. Upjohn International, Kalamazoo, U.S.A., M/s. Laboratoire Choay, Paris, France and M/s. KRKA, Ljubljana, Yugoslavia for security process and technical know-how for manufacturing and marketing some of their products in India.
- Croslands Research Laboratories Limited is being merged with Ranbaxy Laboratories Limited.
- The company recently issued one-for-one bonus shares raising the equity capital to Rs.7.42 crore. Foreign institutional investors (FIIs) hold over 15 per cent of the company's equity. The two promoters hold around 50 per cent, the employees two per cent and the domestic institutions about three per cent.
- The company already has a tie-up with the US-based Eli Lilly, which has helped it to take on the drug multinationals.
- According to Gufic's managing director Jayesh Choksi, Ranbaxy Laboratories has acquired all the major pharmaceutical formulation brands of the Rs.125-crore, Mumbai-headquartered Gufic Labs.
- The annual Ranbaxy research awards were presented to five scientists for their outstanding contributions in medical, pharmaceutical and clinical sciences by Sir Gustav Nossal, immunologist from Australia.
- Dr Sandip Kumar Basu, Director of the National Institute of Immunology (NII), New Delhi, received the prize in the same category for his work on receptor-medicated intracellular delivery of drugs to macrophages, which are specialised cells important for the restoration of damaged cells, and for demonstrating the superior efficacy of these drugs over conventional chemotherapy.
- Ranbaxy Laboratories recently pipped Nicholas Piramal to take over the Mumbai based Croslands Research Laboratories sums up his rationale for merging his 12 year old company with the Rs. 930 crore Ranbaxy Industries.
- RRL has been busy establishing joint ventures, strategic alliances, acquisitions in other countries to gain access to potential global markets. RRL is one of the Indian pharma companies which was able to seize opportunities during the rigid FERA era of the seventies.
- RRL has acquired Ohm Laboratories New Jersey, US and another company Rima Pharmaceuticals in the Ireland to serve the UK market and European Community.
- RRL has resorted to a strategy of combining R&D with marketing probably to introduce drugs through reengineering route. Three 50:50 joint ventures with Eli Lilly and Company of US are planned, one to market products in US, another for R&D in India and yet another one to market Eli Lilly products in India. The objective is to create a large portfolio of Eli Lilly and Ranbaxy products.
- Ranbaxy Laboratories Ltd, Dabur India Ltd and Great Eastern Shipping Company Ltd have signed agreements with NSDL to get their securities admitted for dematerialisation at NSDL.
- Equity shares of Ranbaxy Laboratories Ltd are accepted for dematerialisation at NSDL as their registrar and transfer agents, Karvy Consultancy Services Ltd. have already established electronic connectivity with NSDL.
- Ranbaxy Laboratories has entered into a 50:50 joint venture with Specialty Laboratories Inc, of US to set up a clinical reference laboratory in Mumbai as well as satellite laboratories in Bangalore and Delhi.
- These companies - Delta Drugs Private Ltd, Delta Health Care Private Ltd, Delta Laboratories Private Ltd, Ranbaxy Managers Welfare Ltd and Ranbaxy Employees Welfare Ltd hold an 11.85 per cent stake in the company. In all, Ranbaxy has a share capital of 49.41 million shares.
- The pharma major, Ranbaxy Laboratories, is all set to launch its first investigational new drug (IND) in the U.S. within the next 18 months.
- Shareholders of Croslands Research Laboratories Ltd (CRL) will receive three equity shares of Ranbaxy Laboratories Ltd (RLL) for every seven shares held by them. According to company sources, the High Courts of Punjab, Haryana and Mumbai granted the necessary approvals for the scheme of amalgamation.
- In 1997-98, it entered into a 50:50 joint venture with the New Jersey-based Schein Pharmaceuticals Inc, the generics arm of Bayer AG, Germany, for manufacture of Ranitidine. The company is the first to launch prescription products under its own label in the United States. RLL's chairman and managing director Parvinder Singh has been selected for Business India's businessman of the year 1998 award.
1998
- It has become India's first pharmaceutical company to launch prescription products under its own name and label in the U.S., the world's largest pharmaceutical market with an estimated 1997 sale of $ 101 billions.
- The company has entered the U.S. ethical prescriptions market through Ranbaxy Pharmaceuticals Incorporated (RPI), a wholly-owned subsidiary of the Ranbaxy Laboratories Ltd. The entry has been made with Cefaclor capsules and suspensions.
- Ranbaxy Laboratories promoter Parvinder Singh has sold around 20 lakh equity shares of the company at a price of Rs.650 per share. Over a dozen foreign institutional investors (FIIs) have picked up these shares in a deal struck by JM Financial and Morgan Stanley.
- The board of directors of pharmaceutical giant Ranbaxy Laboratories Ltd has recommended a bonus issue of one share for every share held, subject to the approval of its shareholders.
- Ranbaxy Laboratories Ltd and the All-India Organisation of Chemists and Druggists (AIOCD) have signed a memorandum of understanding for various trade-related issues, including the discount structure for decontrolled products of Croslands, a division of Ranbaxy.
- The $110-bn US insurance major Cigna has revised its memorandum of understanding (MoU) with Ranbaxy Laboratories for general insurance, including health insurance, and has entered into a fresh tripartite MoU which includes Infrastructure Leasing and Financial Services (IL&FS) and Parvinder Singh, chairman, Ranbaxy, in his individual capacity.
- Ranbaxy Laboratories received the National Award 1998' for indigenous development of technology. The company has bagged the award for the third consecutive year.
- Ranbaxy Laboratories Ltd has been ranked among the leading companies in the 1998 edition of `Review 200: Asia's leading companies', the annual survey conducted by Far Eastern Economic Review.
- On 28th Dec, 1998, the company issued bonus share in ratio of one new equity share for every one equity share held.
1999
- Pharma Majors Ranbaxy Laboratories Ltd and Glaxo Ltd announced an agreement for co-marketing of an advanced dosage form of the antibiotic cephalexin.
- According to the co-marketing agreement, Ranbaxy will market the new dosage form as Sporidex AF, while Glaxo extends its phexin line with its innovated product.
- Ranbaxy Laboratories Ltd and Cipla have entered into a strategic partnership to jointly market a select basket of drugs.
- Ranbaxy Laboratories Ltd has signed an agreement with Glaxo for the co-marketing of an advanced dosage form of the antibiotic, Cephalexin.
2000
- The Company has filed an investigational new drug application for its new asthma molecule - Rbx-4638 with the Drugs Controller General of India (DCGI). Ranbaxy is the first company in the country to file an INDA for such a novel drug compound.
- Eli Lilly Ranbaxy, a 50:50 joint venture between Ranbaxy Laboratories and the US-based Eli Lilly & Co., has introduced HumaPen, a new ergonimically designed, resusable insulin delivery service.
- Ranbaxy Laboratories Ltd (RLL) has entered into an agreement with Knoll Pharmaceuticals Ltd (KPL) to market the latter's leading brands in select overseas markets.
- The Company is launching a major supply and sale offensive in the European markets.
- The Company is the eleventh largest company in the international generics market.
- The Company has proposed a stock option scheme for its employees and working directors.
- Ranbaxy Pharmaceutical Inc, the US arm of pharmaceutical major Ranbaxy Labs, has entered into a co-marketing alliance with Purepac to market its generic cardiac drug Enalapril Maleate in the US markets.
- Ranbaxy Laboratories Ltd. has launched its generic Enalapril Maleate tablets in the US, through its wholly-owned subsidiary-Ranbaxy Pharmaceuticals Inc.
- Ranbaxy's late Chairman and Managing Director Parvinder Singh's eldest son Malvinder Mohan Singh is being moved up the ladder in the company as a director.
- Ranbaxy Laboratories and Cipla have expanded an existing partnership by adding one more new drug in their co-marketing arrangement.
- Drug major Ranbaxy Laboratories is setting up a drug discovery laboratory in the US.
- The Company has entered into a marketing alliance with Ajanta Pharma to market some of its natural products in south-each Asia.
- The Company has started a bioinformatics facility on its research and development premises at Gurgaon.
- The Company is set to launch a specialised Website before the actual launch of the Indian version of the wonder drug, Viagra.
- Ranbaxy Laboratories has launched a website www.caverta.com with details of its sildenafil citrate brand.
- Crisil has reaffirmed the P1+ rating assigned to the Rs 130-crore CP programme of the company and the FAAA rating assigned to its fixed deposit programme.
- Ranbaxy Laboratories has been awarded the US Foods and Drugs Administration approval for four key anti-infective products, representing nine formulations.
- Ranbaxy Laboratories is setting up a greenfield manufacturing facility in Vietnam with an investment o fover $10 million to be spent over the next five years.
- In Mar.'2000 it launched CLAFRINAST, this novel drug compound belongs to the VLA (Very Large Antigen)4 class of drug which represents a totally new mechanism for treatment of asthma. No such drug has been launched in the international market.
2001
- Ranbaxy Laboratories is set to reap a windfall after existing the joint venture with the $11 billion US pharma major, Eli Lilly by selling its 50 per cent stake made at an equity investment of Rs 7.2 crore for more than ten times at Rs 78 crore.
-July 3: The US-based Eli Lilly has bought the 50 per cent stake owned by Ranbaxy Laboratories in the joint venture Eli Lilly Ranbaxy for $17 million.
- Ranbaxy Laboratories, on August 17th, received the government's approval to begin selling Cifran-OD, a once-a-day form of the antibiotic ciprofloxacin.
-Ranbaxy Laboratories and Cipla, two of the country's leading pharma firms, on August 27 announced an alliance to co-market the former's once-a-day formulation of ciprofloxacin.
- The US-based Eli Lilly has bought the 50 per cent stake owned by Ranbaxy Laboratories in the joint venture Eli Lilly Ranbaxy for $17 million.
- Ranbaxy has appointed Mr Rashmi Barbhaiya as the head of research and development (R&D) at Ranbaxy.
2002 - Ranbaxy subsidiary receives tentative approval for commercialization of Lisinopril + Hydrochlorothiazine Tablets
- Ranbaxy Laboratories's US subsidiary has received temporary approval for an anti-hypertension drug, Prinizide, issued by US Food and Drug Administration.
- Ranbaxy terminates manufacturing agreement with Eli Lilly
- Ranbaxy Laboratories has recieved approval for launching the anti-asthma compound - Montelukast in India.
- Ranbaxy obtains FDA approval to launch Ceftin
- Ranbaxy obtains exclusive marketing rights for Nifedipine-XL from Penwest, USA
- Ranbaxy Laboratories and Wockhardt on March 7th announced a strategic business alliance for the US, the world's largest market for pharmaceutical products.
- Ranbaxy introduces OTC, herbal remedies
- Ranbaxy's US subsidiary gets USFDA permission for midazolam syrup
- Ranbaxy's subsidiary gets USFDA approval for acetaminophen
- Ranbaxy enters biotechnology arena
-Amarinder Singh resigns from Directorship of Ranbaxy Laboratories.
- Ranbaxy & Schwarz Pharma sign a deal to develop new drug to treat benign Prostate Hyperplasia
- The Ranbaxy Laboratories has got the final approval to manufacture and market cefpodoxime proxetil for oral suspension from the US Food and Drug Administration (USFDA)
- Ranbaxy obtains USFDA approval for Adderall
- 4 Ranbaxy drugs included in WHO list
- Ranbaxy Laboratories Ltd and Procter & Gamble Pharmaceuticals - Germany GmbH (P&G) today (June 26, 2002) announced the execution of an agreement by which Ranbaxy acquires the Brand, Veratidec in Germany from P&G.
- Ranbaxy Lab got regulatory approval for its clinical Phase-I trials of its potentially anti-asthmatic entity
- Ranbaxy makes a breakthrough in its R&D programme
- Ranbaxy garners 90% market share of cefuroxime axetil in the US market
- Ranbaxy gets FDA approval for Isotretinoin
- Ranbaxy gets promotion and marketing rights for Verorab
- FDA approved Ranbaxy Lab's new formulation
- Ranbaxy changes its Asia-Pacific base to Singapore
- Ranbaxy enters into marketing pact with Aventis for Hepatitis vaccine
- Ranbaxy Labs forays domestic OTC segment
- Ranbaxy gets US FDA approval for hypertension drug
- Ranbaxy Lab announces the launch of "Ranbaxy Global Consumer Healthcare"
- Ranbaxy Lab members approve issue of bonus shares
- Ranbaxy files highest number of PCT applications to Wipo
- Ranbaxy gets US FDA permission for Lisinopril
- Ranbaxy Lab got approval by WHO for its anti-AIDs drugs
-T R Mulchandani retires from Directorship of Ranbaxy.
-Ranbaxy Laboratories Ltd, and Synaptic Pharmaceutical Corporation USA today (July 12, 2002) announced the granting of a non exclusive license by Synaptic to Ranbaxy. In exchange for the non-exclusive license; Ranbaxy will provide an up-front licensing fee, milestone payments and royalties to Synaptic if any drugs subject to the license are developed and commercialized.
- Ranbaxy Laboratories Ltd has acquired a liquid manufacturing facility from New York based Signature Pharmaceuticals Inc., on an asset purchase basis through its wholly owned subsidiary Ranbaxy Pharmaceuticals Inc., USA.
-Ranbaxy Laboratories Ltd has informed that Dr J M Khanna, President-R&D and Wholetime Director of the Company has retired on July 31, 2002.
- SRL Ranbaxy, a wholly-owned venture of promoters of Ranbaxy Laboratories has received the prestigeous accreditation from the College of American Pathologists (CAP).
- Ranbaxy receives approval from FDA to market Terazosin Hydrochloride
- Ranbaxy gets US approval to manufacture and market terazosin
- Ranbaxy enters into pact with Korean, Chinese biotech firms
- The project of Ranbaxy Laboratories 'Crusoe,' initiated by Ranbaxy earlier this year, is well on track to result in a saving of Rs 100 crore by the year '03 by way of operational efficiencies in various functional areas.
- Ranbaxy Labs gets US approval for Augmentin Version
- Ranbaxy Laboratories Ltd. (RLL) and Nippon Chemiphar Co. Ltd (NC) and Nihon Pharmaceutical Industry Co. Ltd. (NPI)(a subsidiary of NC) based in Tokyo, Japan announced a business alliance agreement for US$ 50 Bn (JPY 600 billion) pharmaceuticals market in Japan.
- Ranbaxy Laboratories Ltd has informed the Exchange that Ranbaxy SA (Pty) a wholly owned subsidiary of Ranbaxy Laboratories Ltd and Tiger Brands' Healthcare division, Adcock Ingram on October 16, 2002 announced the formation of a 50:50 Joint Venture to exclusively sell and distribute Ranbaxy's range of anti-retroviral products in South Africa
- Ranbaxy Laboratories Ltd has informed the Exchange that it has announced an exclusive licensing agreement with K S Biomedix Ltd (KSB), a U K based bio-pharmaceutical company for marketing TransMID TM
- Ranbaxy Laboratories Ltd's fully owned subsidiary Ranbaxy Pharmaceuticals Inc, has secured the final approval from the US Food and Drug Administration (USFDA) to manufacture and market amoxicillin for oral suspension
2003
- Prof Virander S Chauhan and Dr Kanury V S Rao from the International Centre for Genetic Engineering, and Prof Samir K Brahmachari from the Institute of Genomics and Integrative Biology, all from Delhi, are the seven scientists who bagged Ranbaxy Research Awards for the year 2001.
- Ranbaxy Laboratories has rolled out the GlaxoSmithkline's antibiotic called Augmentin, in the US market
- Ranbaxy Launches next Generation Anti-Retroviral for the first time in India
- Ranbaxy Laboratories Ltd on February 10, 2003 has announced the launch of a high end advanced Cephalosporin, Cefprozil, under the brand name Refzil O
- Ranbaxy obtains USFDA approval to market Augmentin clones
- Ranbaxy gets US FDA nod for Cefadroxil Oral Suspension USP
- Ranbaxy receives tentative approval for manufacturing & marketing of Fluconazole Tablets
- Ranbaxy enters into collaborative research with medicines for development of Anti-Malarial Drug
- Ranbaxy receives USFDA approval to manufacture and Market Ofloxacin Tablets
- Ranbaxy announces FDA approval for the manufacture and commercialisation of Isotretinoin Capsules
- Ranbaxy Laboratories obtains approval from USFDA to manufacture Ganciclovir Capsules
- Ranbaxy receives USFDA approval for AIDS drug
- Ranbaxy Lab gets nod from FDA USA for Mfg, marketing fluconazole
- Ranbaxy Latoratories is at the verge of becoming a Zero-Debt company. The company's secured and unsecured debt is curtailed to less than Rs 7 crore, this will ultimately result in substantial savings in the interest outgo.
- Ranbaxy Laboratories has obtained US Food and Drug Administration (FDA) approval to unveil a new amoxicillin antibiotic drug in US market.
- Ranbaxy receives USFDA approval to manufacture & market Loratadine 10 Mg tablets in the OTC market
- Ranbaxy Labs and Kasturba Medical College owned by the Manipal Group have jointly started a clinical research centre in Manipal.
- Ranbaxy receives USFDA approval to manufacture & commercialize Ofloxacin tablets
- Ranbaxy launches High end anti infective injectable for the first time in India
- Ranbaxy gains USFDA approval for commercialization of Riomet
- Ranbaxy Laboratories has inked an agreement with the Andhra Pradesh Government for starting a research centre in the State.
- Ranbaxy gets FDA approval for diabetes drug
- Company's Animal Health Division signs marketing agreement with Alltech Biotechnology for YeaSacc 1026 Bolus, a highly valued product in the dairy cattle segment
- Ranbaxy Laboratories Ltd and GlaxoSmithkline plc (GSK) have entered into a drug discovery and clinical development collaboration covering a wider range of therapeutic areas.
-United States Food and Drug Administration has permitted Ranbaxy Laboratories Ltd to manufacture and commercialise Metformin HCI oral solution 100 milligram per millilitre.
-The company has received prestigious National Safety Awards for the year 2001 & 2002
-Unleashed a cholesterol-reducing agent called 'Rosuvas (rosuvastatin)', which reduces triglycerides and raises HDL..
-Ranbaxy gets FDA approval to market cephalexin
-Got approval from US FDA for the manufacture and marketing of Merck & Co Inc's cholesterol-lowering drug generic Zocor.
-Company has received tentative approval from the office of Generic Drug of the US Food & Drug Administration to manufacture and market Metformin Hydrochloride extended-release tablets, 500 MG.
-Secured USFDA approval for Simvastatin drug
-Ranbaxy gets approval from US FDA for selling its generic form of Bristol-Myers Squibb's cholesterol-reducing Pravachol.
-Received tentative approval from the office of generic drugs of the US Food and Drug Administration to manufacture and market pravastatin sodium tablets 10mg, 20mg, 40mg, and 80 mg.
-Ranbaxy Laboratories granted first USFDA Approval to manufacture and Market Cefaclor Tablets
2004
-Ranbaxy Laboratories Ltd announced that all the necessary formalities and consents required for the conclusion of the acquisition transaction of RPG (Aventis) SA have been obtained and that the acquisition is now complete. With this, RPG (Aventis) SA France has now become a wholly owned subsidiary of Ranbaxy.
-Ranbaxy receives USFDA approval to commercialise Minocycline Hydrochloride Tablets USP
-Ranbaxy Laboratories Ltd has informed that the Stock Exchange, Ahmedabad has advised delisting the securities of the Company effective January 15, 2004.
- In an attempt to tap the domestic $1 billion herbal medicines market, Ranbaxy Laboratories Ltd on Jan 21 unveiled three new brands under the `New Age Herbals' brand. As part of its expansion in the over-the-counter (OTC) segment, the company has launched Olesan Oil for cold, Olesan cough syrup and Eat Ease, an appetite enhancer. Ranbaxy had forayed into the OTC market through a separate division - Ranbaxy Global Consumer Healthcare (RGCH) in October 2002 and is hoping to launch five to six products this year.
-Ranbaxy Laboratories Ltd at its meeting held on January 23, 2004, has approved the appointment of Mr Gurcharan Das as an Additional Director on the board of directors of the company.
-Ranbaxy Laboratories Ltd has signed a research agreement with the Institute of Nuclear Medical and Allied Sciences (INMAS) for screening and evaluation of formulations and New Drug Delivery Systems (NDDS) using Gamma Scintigraphy.
-Ranbaxy unleashes liquid form of diabetes drug in US market
-Introduces its first branded prescription medicine, Visclair tablets 100 mcg (Mecysteine HCL) in the UK
-Surpasses global sales of $1 billion (February 2004, MAT Basis)
-Launches Forzest (Tadalafil), its most recently approved prescription medicine for the treatment of erectile dysfunction (ED) in men
-Bags tentative USFDA approval to manufacture and market Quinapril Hydrochloride tablets used for the treatment of hypertension
-Delists shares from Ludhiana Stock Exchange
-The outgoing CEO and MD of Ranbaxy, Mr D S Brar, has joined the board of Jerry Rao-promoted Mphasis as an independent director
-Ranbaxy receives Tentative approval to market Fenofibrate tablets
- Ranbaxy Laboratories sets up two more wholly-owned subsidiaries in US taking total number of US subsidiaries to eight from six last year.
-Drug company Ranbaxy Laboratories Ltd said on June 22 that its anti-diabetic drug has got the final approval from regulatory authorities in the US
-Ranbaxy & Atrix sign a licensing Agreement for Prostrate Cancer Drug in India
-DS Brar exits from Ranbaxy Laboratories Ltd
-Ranbaxy's anti-cancer drug Metformin is learnt to have received the final approval from the US Food and Drug Administration (FDA)
-Indian pharma major Ranbaxy Laboratories has received final approval from the US FDA to manufacture and market Fluconazole tablets and oral suspension in varying dosages in the US.
-Ranbaxy ties up with Axis-Shield to cure Rheumatoid arthritis
- Ranbaxy Laboratories Ltd receives approval from U. S. Food and Drug Administration to manufacture and market Loratadine Syrup (Loratadine Oral Solution, USP), 5mg/5ml that is available to patients as an OTC product.
- Receives tentative approval from the US Food and Drug Administration for generic anti-depressant, Gabapentin, in capsule form
-Signs collaborative research agreement in the area of New Drug Discovery research with Avestha Gengraine Technologies Pvt Ltd (Avesthagen)
-Ranbaxy prolongs partnership with Aventis
-Ranbaxy inks pact with Ventiv to market Sotret in US
-Ranbaxy launches Flotral for urological disorder amongst ageing males
-Ranbaxy announces launch of Quinapril Tablets by Teva
2005
- Receivs approval from the U.S. Food and Drug Administration to manufacture and market Clarithromycin XL 1000 mg tablets.
-Ranbaxy in alliance with Novavax to step in to biotech sector
-Ranbaxy Laboratories Ltd enters into collaborative agreement with National Chemical Laboratories, Pune and Department of Science & Technology in the area of New Drug Discovery.
- On May 24, 2005 launches its approved generic formulation of Clarithromycin immediate release (IR) tablets, 250 mg and 500 mg, in the US markets.
-Ranbaxy's Joint Venture with Nippon Chemiphar Ltd launches Vogseal 0.2mg & 0.3mg tablets for Diabetes
-Ranbaxy sets up additional manufacturing facility in Malaysia
-Ranbaxy opens third state of the art R&D facility on Gurgaon campus
2006
-Ranbaxy Laboratories enters into a strategic alliance with Zenotech
-Ranbaxy ties up with Ipca to tap US market
-Ranbaxy signs licensing agreement with Swiss Company Debiopharm, for NCE Drug in the Gastroenterology Segment
-Ranbaxy signs licensing agreement with Swiss Company Debiopharm, for NCE Drug in the Gastroenterology Segment.
-Ranbaxy Appoints Atul Sobti as COO.
-Ranbaxy Laboratories Ltd has announced that the Company has entered into a collaborative agreement with the Department of Science & Technology (DST), Government of India, New Delhi, in the area of New Drug Discovery Research (NDDR).
-Ranbaxy granted final USFDA approval to Market Simvastatin 5, 10, 20 & 40 MG Tablets.
2007
-Ranbaxy Laboratories Ltd and GlaxosmithKline (GSK) on February 06, 2007 have signed a new multiyear R&D agreement that modifies and expands the terms of their strategic alliance established in 2003 to provide the Company expanded drug-development responsibilities and further financial opportunities.
- Ranbaxy unveils asthma inhalation capsules.
- Ranbaxy Laboratories Ltd has received final approval from the U.S. Food and Drug Administration (FDA) to manufacture and market Amlodipine Besylate Tablets, 2.5 mg (base), 5 mg (base) and 10 mg (base).
- Ranbaxy Laboratories Ltd on August 22, 2007 has the launch of Roliflo OD (combination of Tamsulosin and Tolterodine) brand in the Indian market for the management of "bladder outlet obstruction with concomitant overactive bladder", a chronic urological disorder. This is a Novel Drug Delivery System (NDDS) product which is being introduced for the first time in India.
Subscribe to:
Posts (Atom)
